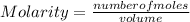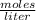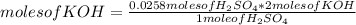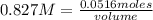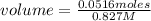Chemistry, 18.11.2020 17:10 ahmedeldyame
In a titration of 35.00 mL of 0.737 M H2SO4, mL of a 0.827 M KOH solution is required for neutralization.
A) 35.0
B) 1.12
C) 25.8
D) 62.4
E) 39.3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
In a titration of 35.00 mL of 0.737 M H2SO4, mL of a 0.827 M KOH solution is required for neutraliz...
Questions

English, 13.04.2020 06:08


Physics, 13.04.2020 06:08

Health, 13.04.2020 06:08


Mathematics, 13.04.2020 06:08

Mathematics, 13.04.2020 06:08

Mathematics, 13.04.2020 06:08





English, 13.04.2020 06:08




Biology, 13.04.2020 06:09

Mathematics, 13.04.2020 06:09