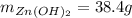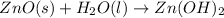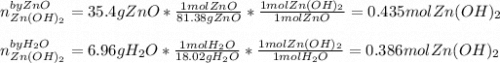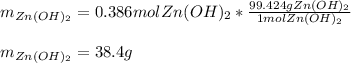Chemistry, 18.11.2020 17:10 kbkbkbkb7611
For the following reaction, 35.4 grams of zinc oxide are allowed to react with 6.96 grams of water . zinc oxide(s) + water(l) zinc hydroxide(aq) What is the maximum mass of zinc hydroxide that can be formed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
For the following reaction, 35.4 grams of zinc oxide are allowed to react with 6.96 grams of water ....
Questions

Mathematics, 24.10.2019 06:43

Mathematics, 24.10.2019 06:43


Chemistry, 24.10.2019 06:43


Biology, 24.10.2019 06:43

Mathematics, 24.10.2019 06:43

Law, 24.10.2019 06:43


Mathematics, 24.10.2019 06:43

Mathematics, 24.10.2019 06:43


English, 24.10.2019 06:43


History, 24.10.2019 06:43

Biology, 24.10.2019 06:43

Mathematics, 24.10.2019 06:43

Mathematics, 24.10.2019 06:43


History, 24.10.2019 06:43