
Chemistry, 18.11.2020 16:50 Conner4600
PLEASEEE HELPPP
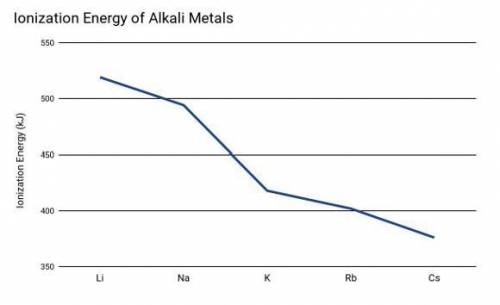
Two students were conducting and experiment based on the ionization energy of alkali metals. The driving question for the experiment was: "What is the relationship between ionization energy and the rate of reaction (time for the reaction to be completed) using alkali metals."
Which of the following predictions would be the best option based on the student's prior knowledge learned and information provided in the graph above?
A.
Cesium will have a higher rate of reaction (faster) because it has the lowest ionization energy which indicates a higher reactivity.
B.
Cesium will have a lower rate of reaction (slower) because it has the lowest ionization energy which indicates a lower reactivity.
C.
The ionization energy of the alkali metals will not affect the rate of the reaction because the energy required to remove an electron does not affect time.
D.
Lithium will have a higher rate of reaction (faster) because it has the highest ionization energy which indicates a higher reactivity.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
PLEASEEE HELPPP
Two students were conducting and experiment based on the ionization energy of alkal...
Questions

Mathematics, 20.11.2019 14:31

Biology, 20.11.2019 14:31

English, 20.11.2019 14:31

Mathematics, 20.11.2019 14:31

Mathematics, 20.11.2019 14:31

Computers and Technology, 20.11.2019 14:31


Health, 20.11.2019 14:31

Mathematics, 20.11.2019 14:31

Biology, 20.11.2019 14:31

Mathematics, 20.11.2019 14:31



Biology, 20.11.2019 14:31



Mathematics, 20.11.2019 14:31


Business, 20.11.2019 14:31

Mathematics, 20.11.2019 14:31



