
Chemistry, 18.11.2020 06:00 nana54muller
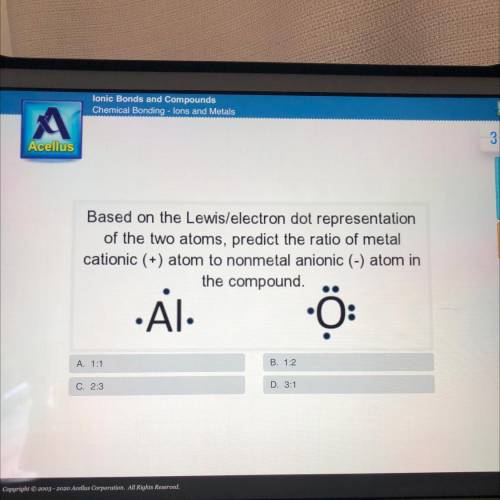
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
cationic (+) atom to nonmetal anionic (-) atom in
the compound.
.Al
A. 1:1
B. 1:2
C. 2:3
D. 3:1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
c...
c...
Questions

Geography, 10.10.2019 14:50


History, 10.10.2019 14:50

Health, 10.10.2019 14:50

History, 10.10.2019 14:50


Mathematics, 10.10.2019 14:50

Social Studies, 10.10.2019 14:50





Biology, 10.10.2019 14:50

Mathematics, 10.10.2019 14:50

Mathematics, 10.10.2019 14:50

Biology, 10.10.2019 14:50



Computers and Technology, 10.10.2019 14:50

Spanish, 10.10.2019 14:50



