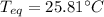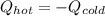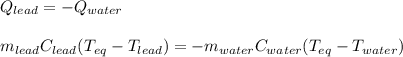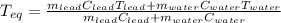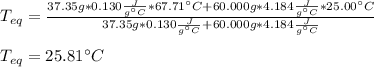Chemistry, 18.11.2020 02:40 diamondk2019
A chemist heats 37.35 g of lead to 67.71 °C , then places the metal sample in the cup of water shown in the interactive. Calculate the final temperature of the water. The specific heat of lead is 0.130 J/g⋅°C and the specific heat of water is 4.184 J/g⋅°C .
mass of water: 60.000g
initial temperature of water: 25.00 C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
A chemist heats 37.35 g of lead to 67.71 °C , then places the metal sample in the cup of water shown...
Questions

Computers and Technology, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20


History, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20

Biology, 22.03.2021 16:20



Mathematics, 22.03.2021 16:20



Mathematics, 22.03.2021 16:20


Mathematics, 22.03.2021 16:20

Social Studies, 22.03.2021 16:20

Engineering, 22.03.2021 16:20