Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

You know the right answer?
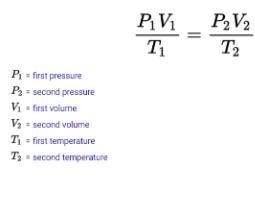
STP= Standard temperature and pressure . standard temperature is 273K and standard pressure is 760 M...
Questions






English, 05.11.2020 03:50


English, 05.11.2020 03:50








Mathematics, 05.11.2020 03:50

Mathematics, 05.11.2020 03:50

Chemistry, 05.11.2020 03:50


Mathematics, 05.11.2020 03:50