
Chemistry, 17.11.2020 17:30 rodriguezbrian050702
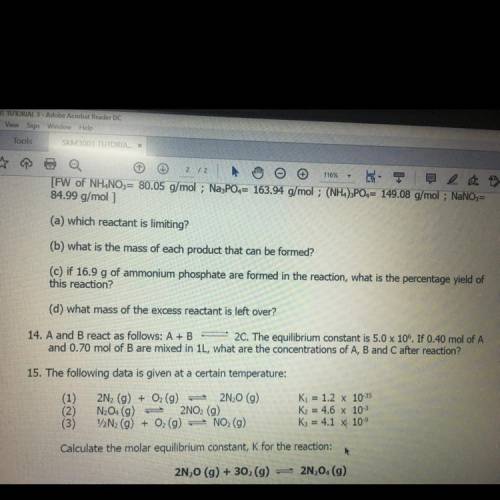
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A and 0.70 mol of B are mixed in 1L, what are the concentrations of A, B and C after reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A
and 0...
Questions

Mathematics, 06.05.2020 03:57

History, 06.05.2020 03:57


Mathematics, 06.05.2020 03:57


Computers and Technology, 06.05.2020 03:57



Advanced Placement (AP), 06.05.2020 03:58

Mathematics, 06.05.2020 03:58

English, 06.05.2020 03:58

Physics, 06.05.2020 03:58

Computers and Technology, 06.05.2020 03:58







Physics, 06.05.2020 03:58



