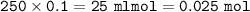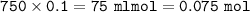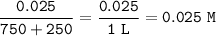Chemistry, 17.11.2020 05:40 tatianna341
Part A A 250 ml sample of 0.10 M Ca(OH)2 is titrated with 0.10 M HCI. Determine the pH of the solution after the addition of 750 ml HCI

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
Part A
A 250 ml sample of 0.10 M Ca(OH)2 is titrated with 0.10 M HCI. Determine the pH of the solut...
Questions


Social Studies, 18.11.2019 10:31

Mathematics, 18.11.2019 10:31





Mathematics, 18.11.2019 10:31


Mathematics, 18.11.2019 10:31

Geography, 18.11.2019 10:31


Mathematics, 18.11.2019 10:31

Advanced Placement (AP), 18.11.2019 10:31





Mathematics, 18.11.2019 10:31