
Chemistry, 16.11.2020 20:20 meganwintergirl
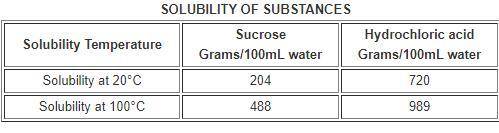
The table below compares the solubility of two substances in 100 milliliters (mL) of water.
a. The solubility of sucrose increases if more is dissolved.
b. The solubility of sucrose decreases as temperature increases
c. The solubility of hydrochloric acid increases if more is dissolved.
d. The solubility of hydrochloric acid increases as temperature increases.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
The table below compares the solubility of two substances in 100 milliliters (mL) of water.
a. The...
Questions

Mathematics, 11.04.2020 04:50






History, 11.04.2020 04:50


History, 11.04.2020 04:50

Computers and Technology, 11.04.2020 04:50












