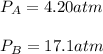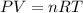Chemistry, 16.11.2020 17:00 noglapotato
A 7.12 L cylinder contains 1.21 mol of gas A and 4.94 mol of gas B, at a temperature of 28.1 °C. Calculate the partial pressure of each gas in the cylinder. Assume ideal gas behavior.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
A 7.12 L cylinder contains 1.21 mol of gas A and 4.94 mol of gas B, at a temperature of 28.1 °C. Cal...
Questions

Chemistry, 31.01.2020 11:51

Health, 31.01.2020 11:51


Mathematics, 31.01.2020 11:51

Mathematics, 31.01.2020 11:51


Mathematics, 31.01.2020 11:51

Mathematics, 31.01.2020 11:51

Mathematics, 31.01.2020 11:51

Biology, 31.01.2020 11:51

Mathematics, 31.01.2020 11:51

Chemistry, 31.01.2020 11:51

Health, 31.01.2020 11:51



Physics, 31.01.2020 11:51