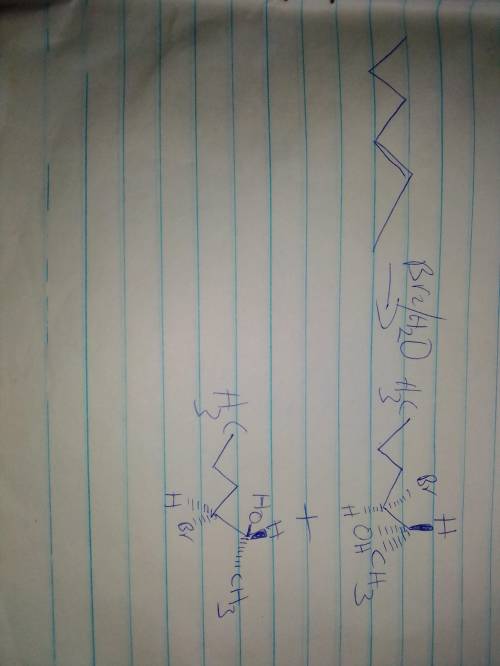
Chemistry, 16.11.2020 17:00 rosemary909
Draw the structure of the bromohydrin formed when (E)-2-hexene reacts with Br, H20.
Use the wedge hash bond tools to indicate stereochemistry where it exists.
• If the reaction produces a racemic mixture, draw both stereoisomers.
Separate multiple products using the - sign from the drop-down menu.
a
С P
opy to
2
2/10
. [+

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
Draw the structure of the bromohydrin formed when (E)-2-hexene reacts with Br, H20.
Use the wedge h...
Questions

Mathematics, 17.10.2020 20:01

English, 17.10.2020 20:01



Mathematics, 17.10.2020 20:01












Mathematics, 17.10.2020 20:01