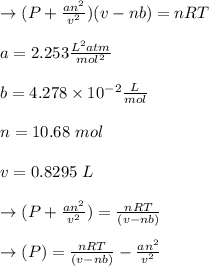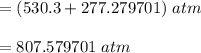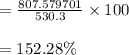Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
According to the ideal gas law, a 10.68 mol sample of methane gas in a 0.8295 L container at 501.9 K...
Questions


Social Studies, 12.12.2020 16:50

Chemistry, 12.12.2020 16:50



Mathematics, 12.12.2020 16:50


Biology, 12.12.2020 16:50

Arts, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Law, 12.12.2020 16:50



Advanced Placement (AP), 12.12.2020 16:50

Mathematics, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50