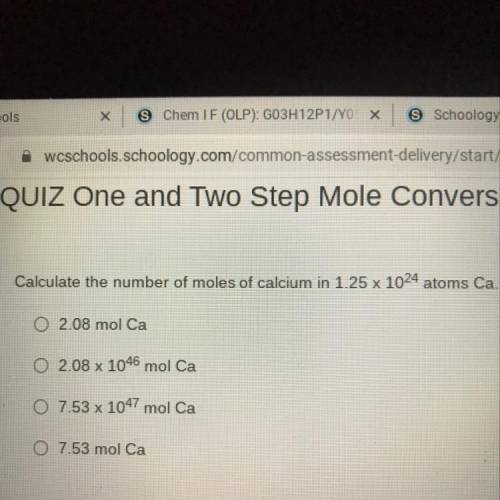
Calculate the number of moles of calcium in 1.25 x 1024 atoms Ca.
02.08 mol Ca
O 2.08 x 1046...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
Questions

Mathematics, 31.08.2020 03:01



Mathematics, 31.08.2020 03:01


Mathematics, 31.08.2020 03:01













Mathematics, 31.08.2020 03:01

History, 31.08.2020 03:01