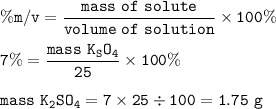Chemistry, 16.11.2020 01:00 calvinclifton
(2 points) How many grams of K2SO4 are present in 25.0 mL of 7.00 % (m/v) solution? Show your work. No work = no credit.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1


Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
(2 points) How many grams of K2SO4 are present in 25.0 mL of 7.00 % (m/v) solution?
Show your work....
Questions



Mathematics, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00



Mathematics, 28.04.2021 01:00


Mathematics, 28.04.2021 01:00

History, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00

Biology, 28.04.2021 01:00




Mathematics, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00

History, 28.04.2021 01:00