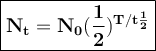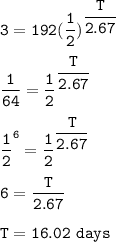Chemistry, 15.11.2020 23:10 elizabethxoxo3271
Yttrium-90, Y90 , is a radioactive isotope used in the treatment of liver cancer. The half‑life of Y90 is 2.67 days. If a dose with an activity of 192 μCi is given to a patient, how many days will it take for the activity of Y90 in the patient to reach 3.00 μCi?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
Yttrium-90, Y90 , is a radioactive isotope used in the treatment of liver cancer. The half‑life of Y...
Questions



Computers and Technology, 22.05.2021 01:00

Physics, 22.05.2021 01:00

Mathematics, 22.05.2021 01:00


English, 22.05.2021 01:00



Chemistry, 22.05.2021 01:00

History, 22.05.2021 01:00

Mathematics, 22.05.2021 01:00

Advanced Placement (AP), 22.05.2021 01:00


Mathematics, 22.05.2021 01:00

English, 22.05.2021 01:00




Mathematics, 22.05.2021 01:00