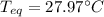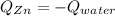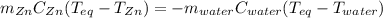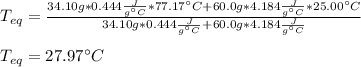Chemistry, 15.11.2020 01:00 george6871
A chemist heats 34.10 g of nickel to 77.17 °C, then places the metal sample in the cup of water shown in the interactive.
Calculate the final temperature of the water. The specific heat of nickel is 0.444 J/g °C and the specific heat of water is
4.184 J/g °C.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2


Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
You know the right answer?
A chemist heats 34.10 g of nickel to 77.17 °C, then places the metal sample in the cup of water show...
Questions


Mathematics, 04.08.2019 23:30




Business, 04.08.2019 23:30

Mathematics, 04.08.2019 23:30


Mathematics, 04.08.2019 23:30

Social Studies, 04.08.2019 23:30

Mathematics, 04.08.2019 23:30

Mathematics, 04.08.2019 23:30

Advanced Placement (AP), 04.08.2019 23:30

Chemistry, 04.08.2019 23:30


Chemistry, 04.08.2019 23:30

Biology, 04.08.2019 23:30

History, 04.08.2019 23:30


Biology, 04.08.2019 23:30