
Chemistry, 14.11.2020 09:40 franvelpaulino2191
A 2.70g vitamin C tablet is found to contain 0.0109 mol of
ascorbic acid (C6H806}. The molar mass of C6H806 is 176.12 g/mol. What
is the mass percent of C6H806 in the tablet?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3


Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1
You know the right answer?
A 2.70g vitamin C tablet is found to contain 0.0109 mol of
ascorbic acid (C6H806}. The molar mass o...
Questions

Mathematics, 19.11.2020 01:50

Mathematics, 19.11.2020 01:50

Mathematics, 19.11.2020 01:50

Mathematics, 19.11.2020 01:50

Mathematics, 19.11.2020 01:50

Mathematics, 19.11.2020 01:50

History, 19.11.2020 01:50

Mathematics, 19.11.2020 01:50


Mathematics, 19.11.2020 01:50

Chemistry, 19.11.2020 01:50

Mathematics, 19.11.2020 01:50


Mathematics, 19.11.2020 01:50


Mathematics, 19.11.2020 01:50


Mathematics, 19.11.2020 01:50

Chemistry, 19.11.2020 01:50

Health, 19.11.2020 01:50

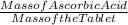 ) * 100
) * 100

