
Chemistry, 13.11.2020 23:20 vanessa791
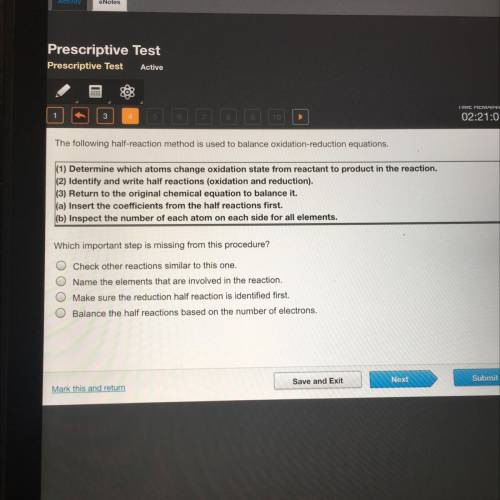
The following half-reaction method is used to balance oxidation-reduction equations.
(1) Determine which atoms change oxidation state from reactant to product in the reaction.
(2) Identify and write half reactions (oxidation and reduction).
(3) Return to the original chemical equation to balance it.
(a) Insert the coefficients from the half reactions first.
(b) Inspect the number of each atom on each side for all elements.
Which important step is missing from this procedure?
Check other reactions similar to this one.
Name the elements that are involved in the reaction.
Make sure the reduction half reaction is identified first.
Balance the half reactions based on the number of electrons.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Type the correct answer in the box. spell all words correctly .what does biodiesel produce in higher amounts? biodiesel produces higher amounts
Answers: 2

Chemistry, 22.06.2019 06:30
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent.true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight.a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
The following half-reaction method is used to balance oxidation-reduction equations.
(1) Determine...
Questions





Biology, 22.12.2019 21:31

History, 22.12.2019 21:31




Mathematics, 22.12.2019 21:31



History, 22.12.2019 21:31


Mathematics, 22.12.2019 21:31

English, 22.12.2019 21:31

Social Studies, 22.12.2019 21:31





