Periodic Trends of the Elements: Tutorial
Warm-Up
The properties of an element depend on the...

Chemistry, 13.11.2020 21:20 princessss30188
Periodic Trends of the Elements: Tutorial
Warm-Up
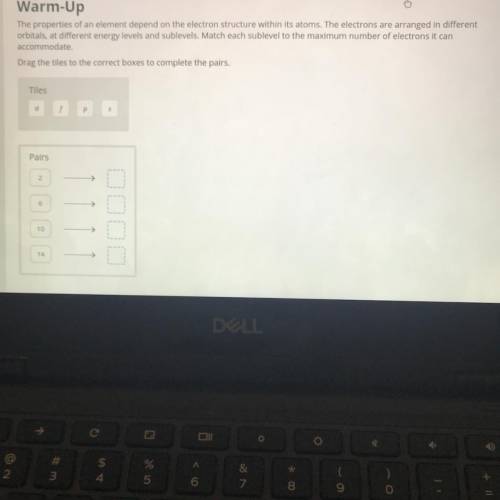
The properties of an element depend on the electron structure within its atoms. The electrons are arranged in different
orbitals, at different energy levels and sublevels. Match each sublevel to the maximum number of electrons it can
accommodate.
Drag the tiles to the correct boxes to complete the pairs.
Tiles
d
Pairs
2
6
10
14

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1
You know the right answer?
Questions







History, 01.02.2021 03:10

Chemistry, 01.02.2021 03:10

Mathematics, 01.02.2021 03:10

Geography, 01.02.2021 03:10


Mathematics, 01.02.2021 03:10


Mathematics, 01.02.2021 03:10

Mathematics, 01.02.2021 03:10


Mathematics, 01.02.2021 03:10

English, 01.02.2021 03:10

History, 01.02.2021 03:10

Biology, 01.02.2021 03:10



