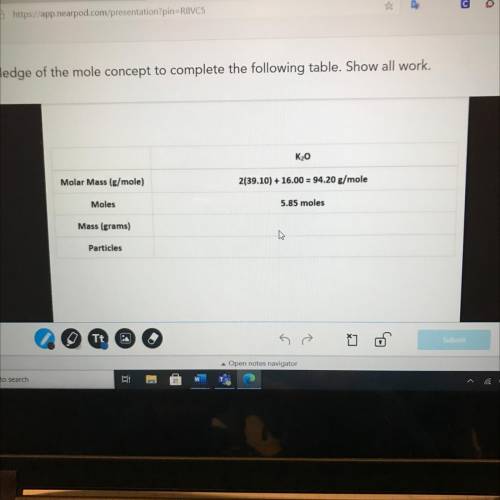
K20
Molar Mass (g/mole)
2(39.10) + 16.00 = 94.20 g/mole
Moles
5.85 moles
Ma...


Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

You know the right answer?
Questions

Social Studies, 12.01.2021 06:00

Mathematics, 12.01.2021 06:00


Social Studies, 12.01.2021 06:00

Chemistry, 12.01.2021 06:00



Mathematics, 12.01.2021 06:00



History, 12.01.2021 06:00

Mathematics, 12.01.2021 06:00


Mathematics, 12.01.2021 06:00