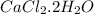Chemistry, 29.08.2019 07:00 mimireds5419
Anhydrous cacl2 is used as a desiccant in desiccators, i. e. it removes water from the air within the desiccators and forms a hydrated salt. a 16.43g mass of anhydrous cacl2 weighed 21.75g after being left in desiccators for several months. what is the formula of the hydrated cacl2?

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1

Chemistry, 23.06.2019 14:00
How does electronegativity changes as we move from left to right across a period
Answers: 2
You know the right answer?
Anhydrous cacl2 is used as a desiccant in desiccators, i. e. it removes water from the air within th...
Questions

Mathematics, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31

Biology, 28.01.2020 12:31


Mathematics, 28.01.2020 12:31


History, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31

Health, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31

Physics, 28.01.2020 12:31

History, 28.01.2020 12:31



Mathematics, 28.01.2020 12:31



English, 28.01.2020 12:31