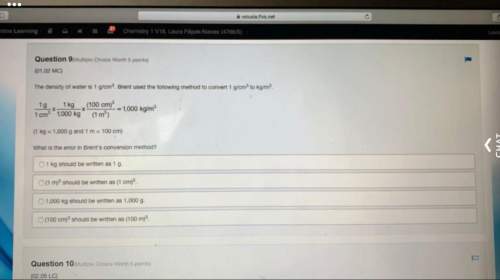
13. The number of unpaired electrons in the complex
[Co(CN)6]3- is
(A) O (B) 1 (C) 2 (D) 4 (E)...

Chemistry, 13.11.2020 01:20 wolfking800
13. The number of unpaired electrons in the complex
[Co(CN)6]3- is
(A) O (B) 1 (C) 2 (D) 4 (E) 3
11
please explain

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Questions



Mathematics, 12.12.2019 00:31


Biology, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31


English, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31



History, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31


Mathematics, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31


Business, 12.12.2019 00:31