Chemistry, 12.11.2020 05:50 F00Dislife
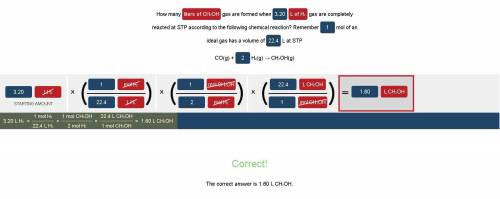
How many liters of CH3OH gas are formed when 3.20 L of H2 gas are completely reacted at STP according to the following chemical reaction?
Remember 1 mol of an ideal gas has a volume of 22.4 L at STP
CO(g) + 2 H₂(g) → CH₃OH(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 19:30
Is the following chemical equation balanced? agno3 + nacl 4agcl + nano3 yes no
Answers: 1
You know the right answer?
How many liters of CH3OH gas are formed when 3.20 L of H2 gas are completely reacted at STP accordin...
Questions




Mathematics, 09.12.2019 00:31



English, 09.12.2019 00:31

Mathematics, 09.12.2019 00:31



English, 09.12.2019 00:31

English, 09.12.2019 00:31

Social Studies, 09.12.2019 00:31

Mathematics, 09.12.2019 00:31


Mathematics, 09.12.2019 00:31


Mathematics, 09.12.2019 00:31


History, 09.12.2019 00:31