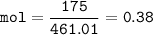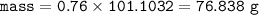Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 06:30
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3

Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1
You know the right answer?
If 175 grams of lead (ll) iodide is produced from the reaction, how many moles of potassium nitrate...
Questions


Biology, 27.01.2021 02:10

Biology, 27.01.2021 02:10


Mathematics, 27.01.2021 02:10


Advanced Placement (AP), 27.01.2021 02:10


Mathematics, 27.01.2021 02:10

Chemistry, 27.01.2021 02:10






English, 27.01.2021 02:10

Health, 27.01.2021 02:10

Mathematics, 27.01.2021 02:10

English, 27.01.2021 02:10