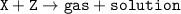Chemistry, 12.11.2020 04:40 tayleeanntabeln2226
A gas is produced when 25 g of substance X is mixed into 60 g of solution Z in a beaker. The mass of the solution in the beaker after the reaction is 75 g. What is the mass of the gas released? (X + Z -> gas + solution)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
A gas is produced when 25 g of substance X is mixed into 60 g of solution Z in a beaker. The mass of...
Questions


Computers and Technology, 04.12.2020 02:50

Mathematics, 04.12.2020 02:50

Mathematics, 04.12.2020 02:50

English, 04.12.2020 02:50

English, 04.12.2020 02:50


Mathematics, 04.12.2020 02:50

Mathematics, 04.12.2020 02:50


Mathematics, 04.12.2020 02:50



Mathematics, 04.12.2020 02:50

Social Studies, 04.12.2020 02:50

Engineering, 04.12.2020 02:50


Mathematics, 04.12.2020 02:50

Biology, 04.12.2020 02:50

English, 04.12.2020 02:50