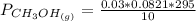Please answer I have a finals and I need help with a question similar!!! Methanol can be synthesized in the gas phase by the reaction of gas phase carbon monoxide with gas phase hydrogen. A 10.0 L reaction flask contains carbon monoxide gas at 0.461 bar and 22.0 °C. 345 mL of hydrogen gas at 7.12 bar and 271 K is introduced. Assume the reaction goes to completion (100% yield). What are the partial pressures of each gas at the end of the reaction, once the temperature has returned to22.0 °C?. units in bar please

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Please answer I have a finals and I need help with a question similar!!! Methanol can be synthesized...
Questions

Advanced Placement (AP), 23.12.2020 21:20

Physics, 23.12.2020 21:20

Mathematics, 23.12.2020 21:20



Mathematics, 23.12.2020 21:20



Arts, 23.12.2020 21:30

Mathematics, 23.12.2020 21:30

Mathematics, 23.12.2020 21:30









Mathematics, 23.12.2020 21:30

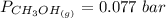
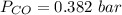
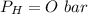

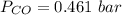
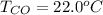
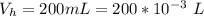
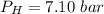
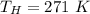
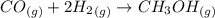
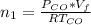
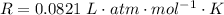
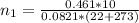
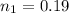
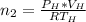
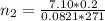
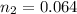
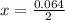
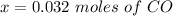

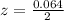
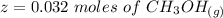
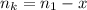
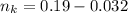
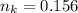
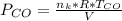
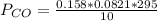
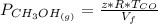
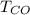 is used because it is given the question that the temperature returned to 22.0 degrees C
is used because it is given the question that the temperature returned to 22.0 degrees C