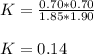Chemistry, 11.11.2020 17:50 karlacarreras152
For the following reaction at equilibrium SO3(g) + NO(g) = SO2(g) + NO2(g)It is found that [SO2] = 0.70 M and [NO] = 1.20 M. Calculate the equilibrium constant for the readction knowing that the initial concentration were [SO3] = 2.55 M and [NO] = 1.90 M.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What phase of matter has particles that are held together but can flow past each other and takes the shape of a container, filling it from the bottom up?
Answers: 1

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1


Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
For the following reaction at equilibrium SO3(g) + NO(g) = SO2(g) + NO2(g)It is found that [SO2] = 0...
Questions

Biology, 16.04.2021 17:50


Biology, 16.04.2021 17:50

Mathematics, 16.04.2021 17:50



Mathematics, 16.04.2021 17:50

History, 16.04.2021 17:50

Mathematics, 16.04.2021 17:50



Mathematics, 16.04.2021 17:50


Mathematics, 16.04.2021 17:50


History, 16.04.2021 17:50


Mathematics, 16.04.2021 17:50

French, 16.04.2021 17:50

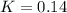
![K=\frac{[SO_2][NO_2]}{[SO_3][NO]}](/tpl/images/0887/9424/17768.png)
 is:
is: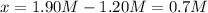
![K=\frac{x*x}{([SO_3]_0-x)([NO]_0-x)}](/tpl/images/0887/9424/71b6f.png)
![[SO_3]=2.55M-0.70M=1.85M](/tpl/images/0887/9424/debed.png)
![[NO]=1.20M](/tpl/images/0887/9424/e6bfc.png)
![[SO_2]=0.70M](/tpl/images/0887/9424/ae391.png)
![[NO_2]=0.70M](/tpl/images/0887/9424/98697.png)