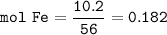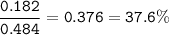Chemistry, 11.11.2020 06:10 connienash95
2. Combining 0.242 mol Fe2O3 with excess carbon produced 10.2 g Fe.
Fe2O3+3C⟶2Fe+3CO
(a) Actual yield of Fe mole
(b) % mole
(c) theoretical yield of iron mmoles

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
You know the right answer?
2. Combining 0.242 mol Fe2O3 with excess carbon produced 10.2 g Fe.
Fe2O3+3C⟶2Fe+3CO
(a)...
(a)...
Questions



Mathematics, 24.03.2020 07:05




Mathematics, 24.03.2020 07:05


Mathematics, 24.03.2020 07:05

Mathematics, 24.03.2020 07:05



Mathematics, 24.03.2020 07:06

Mathematics, 24.03.2020 07:07

Mathematics, 24.03.2020 07:07


Mathematics, 24.03.2020 07:07



English, 24.03.2020 07:07