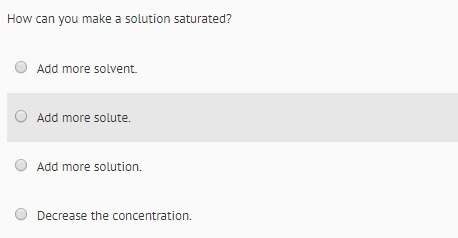
Chemistry, 10.11.2020 21:20 thedeathlord123
Which conclusion could be made from Ernest Rutherford’s gold foil experiment? Atoms are made up of mostly empty space. Atoms are mainly solid and block alpha particles. The volume of the nucleus is large compared to the rest of the atom. The mass of the nucleus is small compared to the rest of the atom.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3


You know the right answer?
Which conclusion could be made from Ernest Rutherford’s gold foil experiment? Atoms are made up of m...
Questions



Mathematics, 05.06.2020 10:57

Mathematics, 05.06.2020 10:57


Mathematics, 05.06.2020 10:57

Health, 05.06.2020 10:57

Mathematics, 05.06.2020 10:57


Mathematics, 05.06.2020 10:57


Mathematics, 05.06.2020 10:57


Mathematics, 05.06.2020 10:57

Mathematics, 05.06.2020 10:57

Mathematics, 05.06.2020 10:57