
Chemistry, 10.11.2020 09:20 Josephjenkins620
The molecular mass of air, at standard pressure and temperature, is approximately 28.97 g/mol.
Calculate the mass of 3.33 moles of air.
First, complete the unit conversion using dimensional analysis:
A • B/C
A: 3.33 mil air
B: 28.87 g air
C: 1 mol air

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1


Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
The molecular mass of air, at standard pressure and temperature, is approximately 28.97 g/mol.
Calc...
Questions

Biology, 30.03.2021 18:40

Social Studies, 30.03.2021 18:40


Mathematics, 30.03.2021 18:40

Arts, 30.03.2021 18:40



Biology, 30.03.2021 18:40

Mathematics, 30.03.2021 18:40

English, 30.03.2021 18:40

Biology, 30.03.2021 18:40


Mathematics, 30.03.2021 18:40

Mathematics, 30.03.2021 18:40


History, 30.03.2021 18:40

Mathematics, 30.03.2021 18:40

English, 30.03.2021 18:40


Mathematics, 30.03.2021 18:40

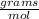 * 3.33 moles
* 3.33 moles

