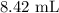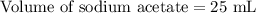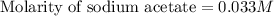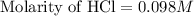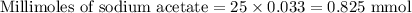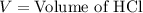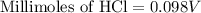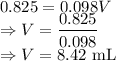Chemistry, 09.11.2020 22:50 deanlmartin
A 25.00 ml solution containing 0.033 M sodium acetate is titrated with a 0.098 M solution of HCl. How mar milliliters of HCl are required to reach the endpoint?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
A 25.00 ml solution containing 0.033 M sodium acetate is titrated with a 0.098 M solution of HCl. Ho...
Questions




Geography, 06.12.2019 16:31



History, 06.12.2019 16:31

Mathematics, 06.12.2019 16:31



English, 06.12.2019 16:31


Mathematics, 06.12.2019 16:31

Mathematics, 06.12.2019 16:31






Mathematics, 06.12.2019 16:31