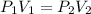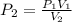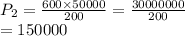Chemistry, 09.11.2020 19:00 rebeccacruzz2017
600. ml of a gas at 50.0 kPa is compressed, at constant temperature, until its volume becomes 200. ml. What is the new pressure of the gas? Be precise! *

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1
You know the right answer?
600. ml of a gas at 50.0 kPa is compressed, at constant temperature, until its volume becomes 200. m...
Questions

Mathematics, 05.07.2019 15:30

Mathematics, 05.07.2019 15:30

Mathematics, 05.07.2019 15:30

Mathematics, 05.07.2019 15:30

SAT, 05.07.2019 15:30

SAT, 05.07.2019 15:30


Advanced Placement (AP), 05.07.2019 15:30

Mathematics, 05.07.2019 15:30

Chemistry, 05.07.2019 15:30




History, 05.07.2019 15:30





Biology, 05.07.2019 15:30