

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
Calculate the pH at 25 degrees celsius of a 0.39 M solution of pyridinium chloride (c5h5nhcl) . Note...
Questions


Mathematics, 31.03.2020 01:08

Mathematics, 31.03.2020 01:08



History, 31.03.2020 01:08




Health, 31.03.2020 01:08



Computers and Technology, 31.03.2020 01:19


Mathematics, 31.03.2020 01:19

Mathematics, 31.03.2020 01:19

Mathematics, 31.03.2020 01:19

Social Studies, 31.03.2020 01:19

Computers and Technology, 31.03.2020 01:19

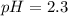
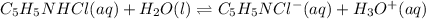
![Ka=\frac{[C_5H_5NCl^-][H_3O^+]}{[C_5H_5NHCl]}](/tpl/images/0880/2037/d1e08.png)
 , we write:
, we write: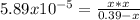
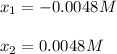
![pH=-log([H_3O^+])=-log(0.0048)\\\\pH=2.3](/tpl/images/0880/2037/04092.png)


