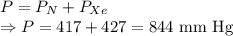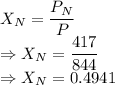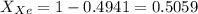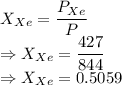Chemistry, 09.11.2020 16:20 allenpaietonp9v8sv
A mixture of nitrogen and xenon gases contains nitrogen at a partial pressure of 417 mm Hg and xenon at a partial pressure of 427 mm Hg. What is the mole fraction of each gas in the mixture

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
A mixture of nitrogen and xenon gases contains nitrogen at a partial pressure of 417 mm Hg and xenon...
Questions

Business, 21.10.2019 21:00

Spanish, 21.10.2019 21:00


Advanced Placement (AP), 21.10.2019 21:00


Mathematics, 21.10.2019 21:00

Biology, 21.10.2019 21:00





Mathematics, 21.10.2019 21:00

Geography, 21.10.2019 21:00

Mathematics, 21.10.2019 21:00



Physics, 21.10.2019 21:00

Biology, 21.10.2019 21:10


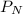 = Partial pressure of nitrogen = 417 mm Hg
= Partial pressure of nitrogen = 417 mm Hg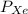 = Partial pressure of xenon = 427 mm Hg
= Partial pressure of xenon = 427 mm Hg