
Chemistry, 09.11.2020 01:50 tristenmathews
Consider the following reaction: 4HCl(g)+O2(g)→2H2O(g)+2Cl2(g) Each of the following molecular diagrams represents an initial mixture of the reactants.(Figure 1) How many molecules of Cl2 would be formed from the reaction mixture that produces the greatest amount of products?
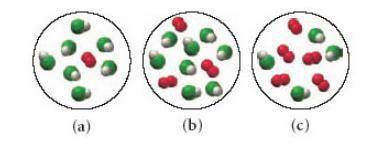

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1
You know the right answer?
Consider the following reaction: 4HCl(g)+O2(g)→2H2O(g)+2Cl2(g) Each of the following molecular diagr...
Questions


Mathematics, 27.01.2021 16:50


English, 27.01.2021 16:50

Biology, 27.01.2021 16:50

Mathematics, 27.01.2021 16:50

Mathematics, 27.01.2021 16:50




Business, 27.01.2021 16:50







Mathematics, 27.01.2021 16:50


Mathematics, 27.01.2021 16:50



