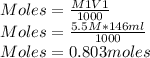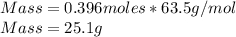Q2. A 0.696 mol sample of Cu is added to 146 mL of 5.5 M HNO3. Assuming the following
reaction is only one that occurs;
Cus) + HNO3(aq) → Cu(NO3)2(aq) + H2O() + NO()
Will the Cu react completely? What is the limiting reagent and what is the remaining compound
in mass?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
Q2. A 0.696 mol sample of Cu is added to 146 mL of 5.5 M HNO3. Assuming the following
reaction is o...
Questions








Mathematics, 24.06.2019 16:00




History, 24.06.2019 16:00

Health, 24.06.2019 16:00


English, 24.06.2019 16:00

Mathematics, 24.06.2019 16:00

Mathematics, 24.06.2019 16:00


English, 24.06.2019 16:00