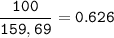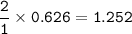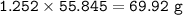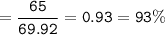Chemistry, 08.11.2020 16:40 athenajames1221
Iron is extracted from iron oxide in the Blast Furnace: Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2
a) Calculate the maximum theoretical mass of iron that can be made from 100g
of iron oxide.
b) In the reaction, only 65 g of iron was made. Calculate the percentage yield.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Iron is extracted from iron oxide in the Blast Furnace: Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2
a) Calculat...
Questions


English, 05.11.2020 23:00

Mathematics, 05.11.2020 23:00


Engineering, 05.11.2020 23:00

History, 05.11.2020 23:00



Mathematics, 05.11.2020 23:00


Mathematics, 05.11.2020 23:00

Mathematics, 05.11.2020 23:00

Mathematics, 05.11.2020 23:00


Engineering, 05.11.2020 23:00

Mathematics, 05.11.2020 23:00

History, 05.11.2020 23:00

History, 05.11.2020 23:00