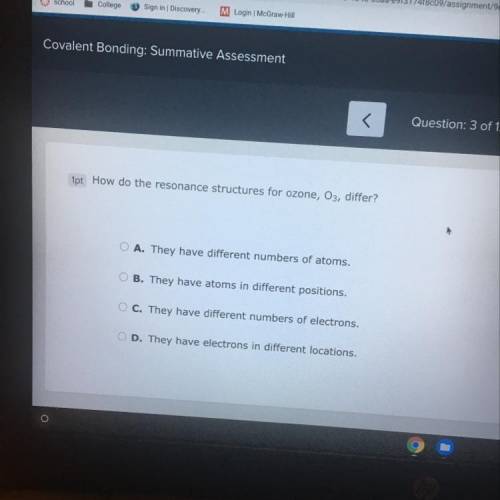
How do the resonance structures for ozone, O3, differ?
A. They have different numbers of atoms...

Chemistry, 07.11.2020 06:20 zulaykaalex
How do the resonance structures for ozone, O3, differ?
A. They have different numbers of atoms.
B. They have atoms in different positions,
C. They have different numbers of electrons.
D. They have electrons in different locations,

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1


Chemistry, 23.06.2019 09:00
How many moles of sulfur dioxide are in 2.26 × 10^33 sulfur dioxide molecules?
Answers: 3

Chemistry, 23.06.2019 11:50
Charles's law describes the relationship of the volume and temperature of gas at a constant mass and pressure. according to this law, what would happen to the temperature of the gas if its volume decreased from 1.0 l to 0.50 l?
Answers: 3
You know the right answer?
Questions

Geography, 25.07.2019 03:30










Social Studies, 25.07.2019 03:30



Business, 25.07.2019 03:30


Computers and Technology, 25.07.2019 03:30

Mathematics, 25.07.2019 03:30





