
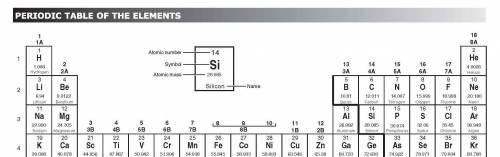
How would an element on the left side of row 2 of the periodic table differ from an element in the middle of the same row?
A. The element on the left would have more atomic mass.
B. The element on the left would have less malleability.
C. The element on the left would have a lower melting point.
D. The element on the left would have no metallic properties.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
How would an element on the left side of row 2 of the periodic table differ from an element in the m...
Questions



Computers and Technology, 31.05.2020 00:01







Mathematics, 31.05.2020 00:01



Mathematics, 31.05.2020 00:01



Mathematics, 31.05.2020 00:02



Mathematics, 31.05.2020 00:02



