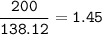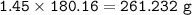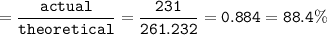PLEASE HELP I HAVE NO IDEA WHAT TO DO
Use the balanced equation below to answer the question. A student begins a reaction with 200.0 grams of C7H603. Calculate the percent yield of the reaction if 231.0 grams of C9H304 is actually
produced.
C7H. O3 + C4H8O3 C9H204 + C2H4O2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
PLEASE HELP I HAVE NO IDEA WHAT TO DO
Use the balanced equation below to answer the question. A stu...
Questions


Chemistry, 16.12.2021 22:20

Chemistry, 16.12.2021 22:20

Advanced Placement (AP), 16.12.2021 22:20







Mathematics, 16.12.2021 22:20

English, 16.12.2021 22:20


Law, 16.12.2021 22:20

Physics, 16.12.2021 22:20


Mathematics, 16.12.2021 22:20


Mathematics, 16.12.2021 22:20