
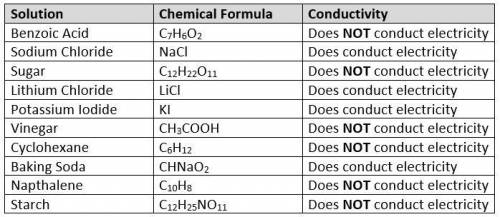
1. Carson completed conductivity testing on the solutions shown in the data table below and recorded his findings in the conductivity column. After determining which solutions conducted electricity and which solutions did not conduct electricity, Carson needs to explain his findings so that he can make predictions about conductivity in the future without having to complete the testing. Why do some of the solutions conduct electricity and some of the solutions not conduct electricity?
Results of conductivity testing: image
Consider Carson's findings, then make a claim to answer the question. Be sure to support your claim with sufficient evidence and reasoning.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

You know the right answer?
1. Carson completed conductivity testing on the solutions shown in the data table below and recorded...
Questions

Mathematics, 21.07.2019 13:30

Computers and Technology, 21.07.2019 13:30


Mathematics, 21.07.2019 13:30

History, 21.07.2019 13:30


Physics, 21.07.2019 13:30

Spanish, 21.07.2019 13:30

English, 21.07.2019 13:30

English, 21.07.2019 13:30




Mathematics, 21.07.2019 13:30

History, 21.07.2019 13:30

Physics, 21.07.2019 13:30

Computers and Technology, 21.07.2019 13:30


Mathematics, 21.07.2019 13:30



