
For many purposes we can treat butane (C4H10) as an ideal gas at temperatures above its boiling point of - 1. °C.
Suppose the pressure on a 3.0 m3 sample of butane gas at 19.0°C is reduced to one-third its initial value.
Is it possible to change the temperature of the butane at the same time such that
the volume of the gas doesn't change?
If you answered yes, calculate the new temperature of the gas. Round your
answer to the nearest °C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2


Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

You know the right answer?
For many purposes we can treat butane (C4H10) as an ideal gas at temperatures above its boiling poin...
Questions

Physics, 04.07.2019 14:10


English, 04.07.2019 14:10





Chemistry, 04.07.2019 14:10

Mathematics, 04.07.2019 14:10

Mathematics, 04.07.2019 14:10


Spanish, 04.07.2019 14:10

Spanish, 04.07.2019 14:10

Mathematics, 04.07.2019 14:10


Mathematics, 04.07.2019 14:10



English, 04.07.2019 14:10

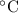 .
.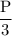
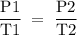
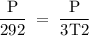
 3T2 = P
3T2 = P 


