
Chemistry, 06.11.2020 17:10 delanieloya
Suppose that 0.410 mol of methane, CH4(g), is reacted with 0.560 mol of fluorine, F2(g), forming CF4(g) and HF(g) as sole products. Assuming that the reaction occurs at constant pressure, how much heat is released

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1

Chemistry, 23.06.2019 07:30
Can you guys answer these questions i need it before 1: 00pm
Answers: 3

You know the right answer?
Suppose that 0.410 mol of methane, CH4(g), is reacted with 0.560 mol of fluorine, F2(g), forming CF4...
Questions

Computers and Technology, 02.11.2020 21:50


Mathematics, 02.11.2020 21:50


Mathematics, 02.11.2020 21:50

Mathematics, 02.11.2020 21:50



Biology, 02.11.2020 21:50


English, 02.11.2020 21:50

Social Studies, 02.11.2020 21:50


Mathematics, 02.11.2020 21:50

Physics, 02.11.2020 21:50


Mathematics, 02.11.2020 21:50



English, 02.11.2020 21:50

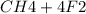 →
→ 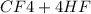
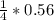 = 0.14mole of CF44 moles of fluorine → 4 moles of HF0.56 mole of fluorine → 0.56 mole of HF
= 0.14mole of CF44 moles of fluorine → 4 moles of HF0.56 mole of fluorine → 0.56 mole of HF

