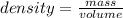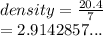Chemistry, 05.11.2020 22:00 dyllanmasters99
A student needs to determine the density of an unknown rock sample by dropping the rock into a graduated cylinder containing water. The original volume in the graduated cylinder is 23.0 ml. The student then drops the rock sample into the graduated cylinder and determines the new volume to be 30.0 ml. If the mass of the rock is determined to be 20.4 grams, what is the density of this sample?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
A student needs to determine the density of an unknown rock sample by dropping the rock into a gradu...
Questions



Mathematics, 17.10.2020 22:01


Mathematics, 17.10.2020 22:01



Mathematics, 17.10.2020 22:01


Mathematics, 17.10.2020 22:01

Mathematics, 17.10.2020 22:01




Mathematics, 17.10.2020 22:01



History, 17.10.2020 22:01