
Chemistry, 05.11.2020 16:40 MendesMist
Carbon monoxide and molecular oxygen react to form carbon dioxide. A 50.0 L reactor at 25.0 oC is charged with 1.00 bar of CO. The gas is then pressurized with O2 to give a total pressure of 3.52 bar. The reactor is sealed, heated to 350 oC to drive the reaction to completion, and cooled back to 25.0 oC. Compute the final partial pressure of each gas (in bar).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
Carbon monoxide and molecular oxygen react to form carbon dioxide. A 50.0 L reactor at 25.0 oC is ch...
Questions


Mathematics, 21.10.2020 03:01




Mathematics, 21.10.2020 03:01

Computers and Technology, 21.10.2020 03:01


Mathematics, 21.10.2020 03:01

History, 21.10.2020 03:01


Mathematics, 21.10.2020 03:01



Mathematics, 21.10.2020 03:01

Health, 21.10.2020 03:01



Mathematics, 21.10.2020 03:01

History, 21.10.2020 03:01

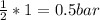 (required)
(required)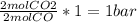 of CO2 2.52 bar O2 (initially) - 0.5 bar (reacted) = 2.02bar O2
of CO2 2.52 bar O2 (initially) - 0.5 bar (reacted) = 2.02bar O2

