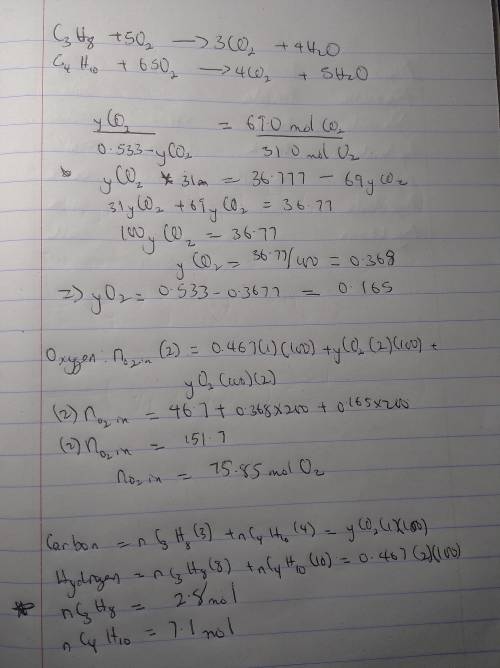Chemistry, 04.11.2020 19:00 tommyaberman
A mixture of propane and butane is burned with pure oxygen. The combustion products contain 46.7 mole% H2O. After all the water is removed from the products, the residual gas contains 69.0 mole% CO2 and the balance O2 a. What is the mole percent of propane in the fuel?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
A mixture of propane and butane is burned with pure oxygen. The combustion products contain 46.7 mol...
Questions

English, 13.10.2020 07:01






Mathematics, 13.10.2020 07:01

Mathematics, 13.10.2020 07:01








Mathematics, 13.10.2020 07:01


English, 13.10.2020 07:01

Physics, 13.10.2020 07:01

Mathematics, 13.10.2020 07:01