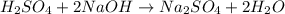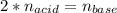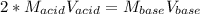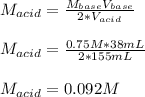Chemistry, 04.11.2020 19:00 joylsbarbour
It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuricacid solution (H2SO4). a. Write a balanced equation for the neutralizationof NaOH with H2SO4

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
You know the right answer?
It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuricacid solution (H...
Questions


English, 15.03.2020 02:15

Mathematics, 15.03.2020 02:15

Mathematics, 15.03.2020 02:15


Mathematics, 15.03.2020 02:16


Mathematics, 15.03.2020 02:16

Mathematics, 15.03.2020 02:16



Mathematics, 15.03.2020 02:17


Mathematics, 15.03.2020 02:17


Mathematics, 15.03.2020 02:18


Mathematics, 15.03.2020 02:18


Mathematics, 15.03.2020 02:19