
Chemistry, 04.11.2020 08:10 smartowl101
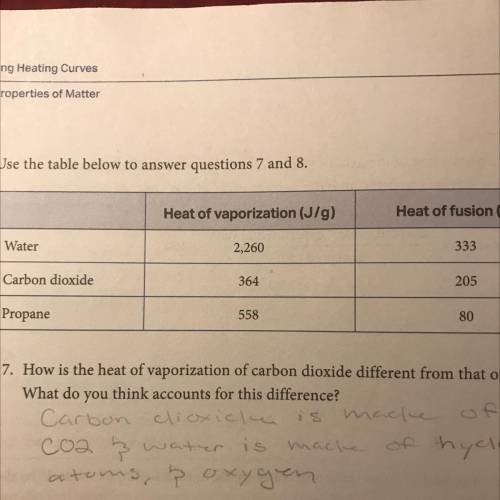
For water, propane, and carbon dioxide, compare the heat of fusion to the heat of
vaporization. What patterns do you see? Do you think these patterns hold true for
other substances as well? Why is vaporization greater than fusion in every case?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

You know the right answer?
For water, propane, and carbon dioxide, compare the heat of fusion to the heat of
vaporization. Wha...
Questions






History, 24.05.2020 05:58

Mathematics, 24.05.2020 05:58



Biology, 24.05.2020 05:58




History, 24.05.2020 05:58

Biology, 24.05.2020 05:58

Chemistry, 24.05.2020 05:58

Mathematics, 24.05.2020 05:58

Chemistry, 24.05.2020 05:58


Chemistry, 24.05.2020 05:58



