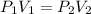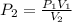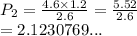Chemistry, 04.11.2020 01:00 Yangster9305
If you have a 4.6 L of gas in a piston at a pressure of 1.2 atm and compress the gas unit it's volume is 2.6 L, what will the new pressure inside the piston be?
21.2 atm
2.12 atm
0.212 atm
0.00212 atm

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1


Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
You know the right answer?
If you have a 4.6 L of gas in a piston at a pressure of 1.2 atm and compress the gas unit it's volum...
Questions

Mathematics, 29.09.2020 01:01



History, 29.09.2020 01:01

History, 29.09.2020 01:01





Geography, 29.09.2020 01:01

English, 29.09.2020 01:01

Engineering, 29.09.2020 01:01




Mathematics, 29.09.2020 01:01


Biology, 29.09.2020 01:01