
Chemistry, 04.11.2020 01:00 AbigailHaylei
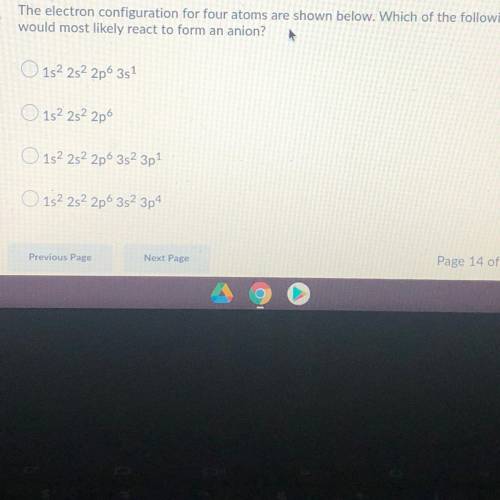
The electron configuration for four atoms are shown below. Which of the following
would most likely react to form an anion?
O 152 252 2p 3s1
152 252 2p
O 152 252 2p6 352 3p
152 282 2p 352 3p4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3


Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
The electron configuration for four atoms are shown below. Which of the following
would most likely...
Questions


Mathematics, 21.11.2020 16:50

Mathematics, 21.11.2020 16:50




Physics, 21.11.2020 16:50

Mathematics, 21.11.2020 16:50

Mathematics, 21.11.2020 16:50


Mathematics, 21.11.2020 16:50

Chemistry, 21.11.2020 16:50

Mathematics, 21.11.2020 16:50


History, 21.11.2020 16:50

Physics, 21.11.2020 16:50

Social Studies, 21.11.2020 16:50

Mathematics, 21.11.2020 16:50

Business, 21.11.2020 16:50

Chemistry, 21.11.2020 16:50



